A promising new Alzheimer’s drug might assist spell “the beginning of the end” for the neurodegenerative illness.
US pharmaceutical large Eli Lilly will announce the complete scientific trial outcomes for its Alzheimer’s drug donanemab at a convention in Amsterdam as we speak.
The drug, which is taken as a month-to-month infusion into the bloodstream for 18 months, was discovered to gradual psychological decline by 36% in part 3 trials, the corporate introduced in May.
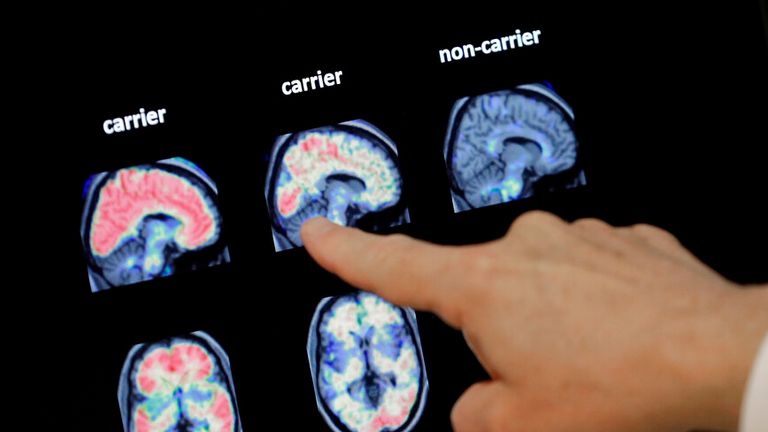
It works by concentrating on and eradicating clusters of the protein amyloid within the mind.
“After 20 years with no new Alzheimer’s disease drugs in the UK, we now have two potential new drugs in 12 just months,” Dr Richard Oakley, affiliate director on the Alzheimer’s Society, wrote in The Mirror.
“This could be the beginning of the end for Alzheimer’s disease.”
It comes after trials confirmed one other drug known as lecanemab slowed development of Alzheimer’s signs by 27% in sufferers within the early phases of the illness. The drug was authorized to be used within the US earlier this month.
After the outcomes of the donanemab trial are launched, specialists world wide will research the findings to look at the advantages of the drug and whether or not they outweigh the danger of unwanted effects.
Such danger components might embrace mind swelling and bleeding.
Regulators within the UK would want to evaluate the drug to resolve whether or not to licence it as secure – a course of that might take between 12 and 18 months – then the NHS would resolve which sufferers it may be prescribed to primarily based on cost-effectiveness.
Estimates recommend the medication might assist 720,000 folks, in accordance with the Alzheimer’s Society and Alzheimer’s Research UK, together with 286,000 with gentle Alzheimer’s and 435,000 with gentle cognitive impairment, which is a precursor to the illness.
Is UK prepared for brand spanking new Alzheimer’s medication?
However, docs beforehand warned 1000’s of sufferers within the UK might miss out on the advantages if such medication grow to be obtainable as a consequence of an absence of mind scanners and specialist clinics.
Amyloid plaques might be detected by taking a pattern of spinal fluid or by a PET scan, of which there are round 86 scanners within the UK.
Click to subscribe to the Sky News Daily wherever you get your podcasts
Around 850,000 folks in Britain have Alzheimer’s illness, which is the most typical type of dementia.
It causes gentle reminiscence loss and might significantly have an effect on an individual’s capability to hold out bizarre every day actions.
Content Source: information.sky.com